

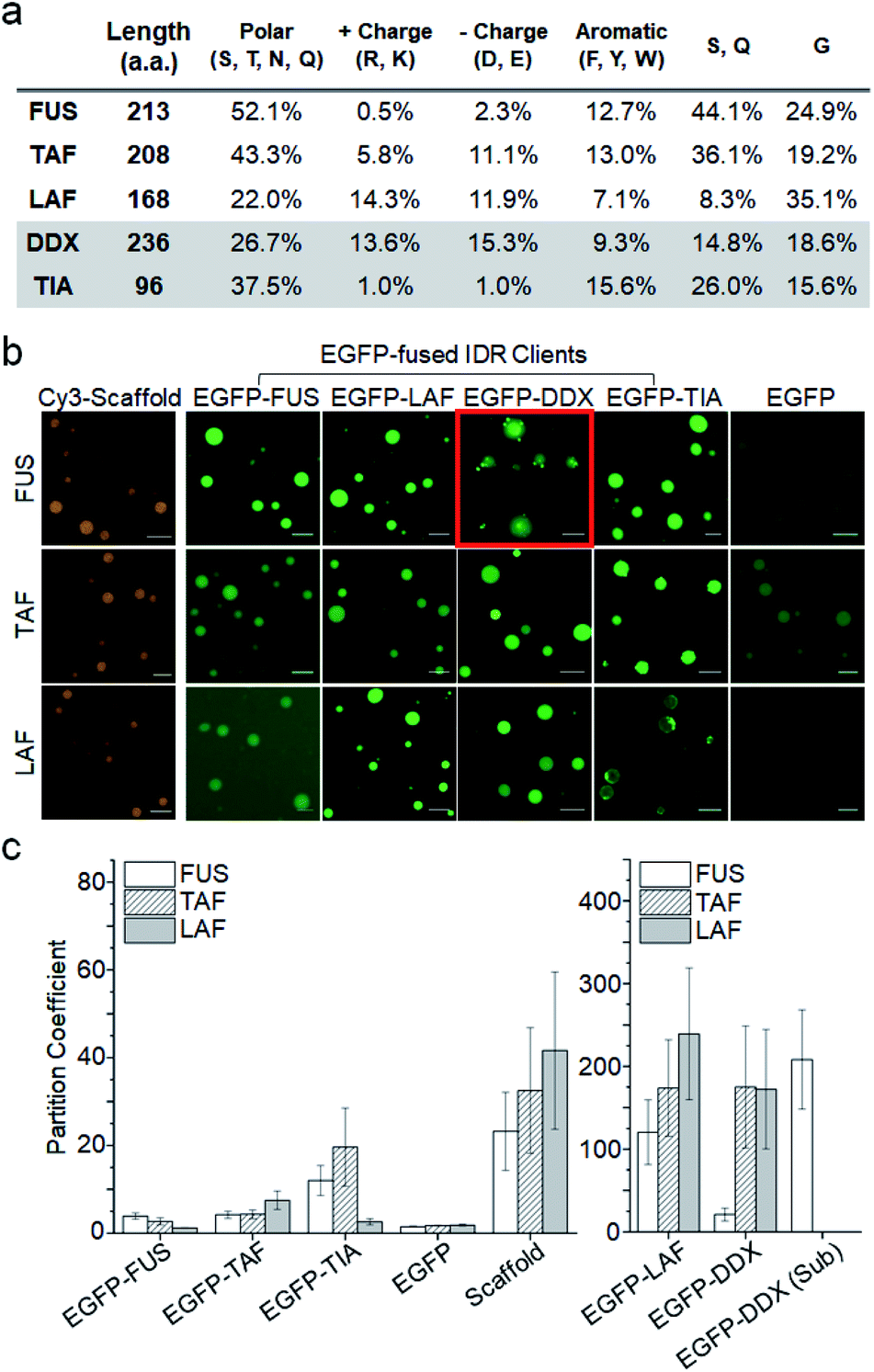
For example, ribozyme cleavage was enhanced 70-fold by RNA enrichment (~3000-fold concentration increase) in the dextran-rich phase of a polyethylene glycol (PEG)/dextran aqueous two-phase system 10. Several studies reported enhanced biomolecular reactions by increased reactant concentrations inside synthetic model compartments 10– 16. Other components are rather passively recruited into the condensates and hence called clients.Īlthough more information on the acting mechanisms of biomolecular LLPS is continuously being revealed, our understanding of biomolecular behaviors such as protein interactions and enzymatic reactions inside membrane-less compartments is still very limited. Among numerous components of membrane-less organelles, a small number of IDPs (sometimes even a single IDP) have been shown to be necessary and sufficient to form condensates, both in vitro and in cells 1– 3, and are termed scaffolds. Recent studies indicate that repeated folded protein domains 4 or intrinsically disordered proteins/regions (IDPs/IDRs) 5– 7 can drive liquid–liquid phase separation (LLPS), and that this is the major formation principle of compartmentalized biomolecular condensates (also termed droplets) 2, 8, 9.
Fus protein scaffold and client free#
Many membrane-less compartments show remarkable liquid-like properties such as high inner diffusivity, reversible formation, and free (yet possibly controlled) exchange of molecules with their surroundings 3. Examples of these membrane-less organelles include stress granules, p-bodies, and nucleoli, which are known as essential hubs of cellular processes such as signal transduction, stress response, and gene expression 2. In addition to conventional membrane-bound organelles such as the endoplasmic reticulum or Golgi, many membrane-less compartments, which are condensed with distinct sets of biomolecules without discrete lipid bilayer barriers (therefore also termed biomolecular condensates), have been reported 1. We propose that two aspects should be considered when explaining client proximity enhancement by phase separation compartmentalization: (1) clients are selectively recruited into compartments, leading to concentration enrichment, and more importantly, (2) recruited clients are further localized around compartment-forming scaffold protein networks, which results in even higher client proximity.Įukaryotic cells utilize various interior compartments to control highly complex biomolecular reactions in space and time. By employing an in vitro phase separation model, we discovered that the operational proximity of clients (measured from client–client interactions) could be over 16 times higher than the expected proximity from actual client concentrations inside compartments. Under a wide range of phase separation conditions, protein interaction signals were vastly increased only inside compartments, indicating greatly enhanced proximity between recruited client proteins. Here we report quantitative measurements of changes in protein interactions for the proteins recruited into membrane-less compartments (termed client proteins) in living cells. Although their formation mechanisms have been steadily elucidated via the classical concept of liquid–liquid phase separation, biomolecular behaviors such as protein interactions inside these liquid compartments have been largely unexplored.
Membrane-less organelles or compartments are considered to be dynamic reaction centers for spatiotemporal control of diverse cellular processes in eukaryotic cells.


 0 kommentar(er)
0 kommentar(er)
